Research Article
Reproductive Characteristics of Leuciscus vorax (Heckel, 1843) from Al-Huwaiza Marshes Southern Iraq 
 Author
Author  Correspondence author
Correspondence author
International Journal of Marine Science, 2017, Vol. 7, No. 47 doi: 10.5376/ijms.2017.07.0047
Received: 27 Oct., 2017 Accepted: 24 Nov., 2017 Published: 15 Dec., 2017
Abdullah A.H.J., Zaidy F.M.A., and Habbeb F.S., 2017, Reproductive characteristics of Leuciscus vorax (Heckel, 1843) from Al-Huwaiza Marshes southern Iraq, International Journal of Marine Science, 7(47): 447-454 (doi:10.5376/ijms.2017.07.0047)
A total of 114 (76 female and 38 male) specimens of Shelig Leuciscus vorax were monthly collected from Al-Huwaiza marsh southern Iraq from November 2016 to October 2017. The samples were caught utilizing gill net and electrofishing. The highest values of gonadosomatic index (GSI) were found in February (18.35 and 5.33 for female and male respectively). Only mature individuals of L. vorax female were used to estimate absolute-relative fecundity and measure eggs diameters of very ripe gonads. Absolute fecundity ranged from 77612 eggs at 56 cm in total length (1672 g) body weight to 378391 eggs 79.5 cm in total length and (5001 g) boy weight. Highly significant differences relationships were observed among to total length, body weight and reproductive parameters. The relative condition factor differs from 0.85±0.11 in October to 1.37±0.09 in June. The results showed one spawning peak on reproductive strategy of represented species, which is preceding other species of fish to enables the small fish of L. vorax feeding on larvae and other small fish species in assemblage.
Background
The Leuciscus vorax is highly commercial native cyprinid species inhabit Euphrates–Tigers Rivers, and is the only representative of this genus in the Tigris and Euphrates Basin (Coad, 2010; Oymak et al., 2011).
The reproduction is an important stage in life of organism in order to preserve the survival and continuity of the species. Understanding of reproductive strategies of fishes and the evaluation of fecundity are indispensable themes in the investigation of the biology furthermore assemblage dynamics of fish species (Hunter et al., 1992). The study of reproduction biology have a high benefits in fishes resource management and develop laws that regulate their properly investment, although our fish stocks are constantly deteriorating due to several factors: overfishing, pollution, lack of water discharges, fragmentation of environments and an increase in the number of exotic introduce species (Dudgeon et al., 2006).
Fecundity, usually refers to potential annual fecundity, its specific to species and can be defined; the number of ripe eggs that laying per female of fish during one spawning season (Murua et al., 2003), which differ from few eggs to millions in some species that spawn fish laying their eggs in open water on an annual basis. However; fecundity are inversely related with the amount of parental care among species in reproductive traits: Viviparous fishes have lower fecundity than ovoviviparous fishes, which in turn have lower fecundity than oviparous fishes, and nest builders have lower fecundity than pelagic spawners (George, 1981).
Due to the lack of studies in southern Iraq on this economic species, this study was executed.
Several studies have been done on reproductive feature of L. vorax in Euphrates-Tigris Rivers Basin (Shafi and Jasim, 1982; Ali et al., 1986; Al-Dabical and Al-Daham, 1995; Epler et al., 2001; Szypuła et al., 2001; Al-Selah et al., 2010; Al-Tameemi et al., 2010; Oymak et al., 2011; Al-Saleh et al., 2012; Duman and Gül, 2013).
The aim of this study was to acquire insights on such Gonadosomatic index (GSI), Absolute fecundity, Relative fecundity and condition factor. The information obtained from this study could reinforce the knowledge of the reproductive biology of L.vorax collected from Al-Huwaiza marshes South of Iraq.
1 Materials and Methods
A total of 114 specimens (76 female and 38 male) were monthly collected from Al-Huwaiza marshes N31º27′, E47º39′ during the period from November 2016 to October 2017. Fishes were caught by gill net (47- 55mm mesh size), and electrofishing. Samples were brought to the laboratory, and total length was measured in centimeters to the nearest 0.1 cm, body weight to nearest 0.1 g, ovary weight to the nearest 0.1 g, ovary length to nearest 0.1 cm and egg diameter in micron (µ) of mature specimens. Fish identification was done due to Coad (2010).
1.1 Gonadosomatic Index (GSI)
Determined by tested the gonads of male and female twice in month and calculated according to Lagler (1966).
GSI%=GW/Body weight (g) *100
Where GW represented gonads weight (g).
Absolute fecundity was calculated using gravimetric methods (Bagenal, 1978). Ovary of ripe females were removed, subsamples of fresh ovary eggs were taken from the front, middle and from the back of ovaries and then counted. The total number of eggs per female was obtained which represented absolute fecundity. Relative fecundity calculated by the equation:
Relative fecundity=Absolute fecundity/ body weight (g)
Eggs diameter were measured of oocytes by objective micrometer (µ).
Morphometric and reproductive parameter relationships have been done using the formula of Nikolsky (1969).
Y= axb, i.e., Log Y= loga+blogx
The calculated weight was obtained from the weight –length relationship and we can express as:
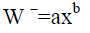
Where W ˉ is the calculated weight (g), X is the total length, (a) and (b) are constants.
Relative condition factors (Kn) were estimated by applying the formula of Dar et al. (2012).
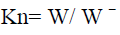
Where W is the observed weight (g), W ˉ is the calculated weight (g).
Statistical analysis were performed by used SPSS software program ver. 20 to analysis the correlation coefficient.
2 Results
Gonadosomatic index (GSI) exhibited monthly changes along the time over the year. The GSI values take the gradual rise in the female from November 2016 as 1.94 to attain the peak 18.31 in February 2017, then started decrease to 1.87 after that continued to increase. In the males the lowest value 0.67 in April and continued rise to the highest to attain 5.33 in February 2017 then started to decrease again (Figure 1). Significant differences were detected in GSI values between females and males.
|
Figure 1 Monthly variations in gonadosomatic index (GSI) of Leuciscus vorax collected from Al-Huwaiza marshes south of Iraq during November 2016 to October 2017 Note: Error bars are standard deviation |
The ovaries of L. vorax occupy the entire body cavity before the spawning take place for several days. The two ovary lobes were often heterogeneous in size, length and weight. The lobe of the ovary has irregular shape; the middle portion was bigger than upper and lower section. The examined of ovaries shown that the shape, size and color variable in the different stages of maturity. When the eggs were very ripe take a yellowish color and easy to release from the ovary.
The fecundity in fishes was rise due to increase in length, weight, status of fish, food available and environmental condition Table 1. The total length of sampled specimens ranged from 55 to 79 cm in total length. Absolute fecundity ranged from 77612 eggs at 56 cm total (1672 g body weight) to 378391 eggs 79.5 cm (5001 g). Relative fecundity ranged from 46.42 to 120.77. Ovary weight oscillated between 148.08 to 762 g. The ovary length varied from 23.20 to 38.50 cm. Eggs diameter were fluctuated from 1624 to 2015 µ for total length differ for 70.3 to 58.1 cm, respectively.
|
Table 1 Reproductive and morphometric features female of Leuciscus vorax fish collected from Al- Huwaiza marshes South of Iraq during November 2016 to October 2017 |
Strong positive significant correlation coefficient (r=0.91) (P<0.01) was showed between absolute fecundity and total length relationship (Table 2; Figure 2). The equation was to be liner and can addressed as:
Log AF=-2.3742+4.2492 log TL
Where AF=absolute fecundity and TL=total length (cm).
|
Table 2 The relationship between reproductive and morphometric features of Leuciscus vorax female from Al-Huwaiza marshes south of Iraq during November 2016 to October 2017 Note: Abscissa (x); Y-intercept (a); Slope (b); correlation coefficient (r) *=Significant at level (0.01) |
.png) Figure 2 The relationship between absolute fecundity and total length, body weight, ovary weight, ovary length, ova diameter and body weight and total length of Leuciscus vorax female during November 2016 to October from Al-Huwaiza marshes |
The relationship between Absolute fecundity and body weight was revealed high positive significant correlations (r= 0.88) (P<0.01), the equation can be expressed as:
Log AF=0.5926+1.3866 log BW
Where AF= absolute fecundity and BW=body weight (g).
The relationship between Absolute fecundity and ovary weightshowed a highly positive significant correlation (r=0.87) (P<0.01) and can be expressed:
Log AF=3.287+0.7961 log OW
Where AF= absolute fecundity and OW= ovary weight (g).
Medium positive correlation coefficient (r= 0.698) (P<0.05) was found between relationship of absolute fecundity and ovary length (cm) and can be addressed as:
Log AF=2.045+2.2324 log OL
Where AF= absolute fecundity and OL= Ovary length.
Weak correlation coefficient (r=0.254) (P<0.05) was detected between the relationship of absolute fecundity and ova diameter (µ) and can be expressed as:
Log AF=11.803-2.0125 log OVD
Where AF= absolute fecundity and OVD= ova diameter.
A strong positive correlation coefficient (r=0.987) (P<0.05) was discovered between the relationship of body weight and total lengthand the equation can be addressed as:
Log W=-1.9633 + 2.9666 log TL
Where W= weight (g) and TL= total length (cm).
The relative condition factor mean values of L. vorax female ranged from 0.85±0.11 in October to 1.37±0.09 in June of mean total length 41±4.32 to 50.3±1.636 cm respectively. However; the means of weight varied from 631.3 ±268.40 to in October to 1719.7 ± 56.002 g in June (Figure 3).
|
Figure 3 Monthly variations in condition factor of Leuciscus vorax female from Al-Huwaiza marshes during November 2016 to October 2017 |
3 Discussion
Temperature is fundamental factor stimulating to acceleration the gonads maturity in fishes (Pankhurst and King, 2010). The results in the present work appear that maximum values of GSI for female and male occurred in February (Figure 1), Shafi and Jasim (1982) pointed that L. vorax spawning started in January at Habania lake, whereas Epler et al. (2001) reported that spawning started in February in Habania and Tharthar in Iraq, while Al-Saleh et al. (2012) stated that A. vorax spawning within February to March in the Euphrates River in Syria. However; the spawning season in the North sector of Euphrates-Tigris Basin in Turkey from April to May and that combatable with the results of Oymak et al. (2011). Gonadosomatic index (GSI) is a good indicator to determine the spawning time in fishes, and common parameter widely used by the biologists to predict of spawning season of fish (Alam and Pathak, 2010). The variation in spawning time attributed to poikilotherms, due to variance of geographical laccolites which play an important role in spawning time (Wright and Trippel, 2009; Pankhurst and King, 2010). The results showed one spawning peak on reproductive strategy of represented species, which is preceding other species of fish to enables the small fish of L. vorax feeding on larvae and other small fish species in assemblage.
Variations in fecundity among the same and different species are common phenomenon and differ from year to other Najim et al. (2012). Many factors could be influenced fecundity; length, weight, age, food abundance, environmental factors and fish status (Lambert et al., 2003; Kamler, 2005). However, there is compatible with our finding in the present work in general trends and with the previous studies on represented species (Al-Selah et al., 2010; Oymak et al., 2011; Al-Selah et al., 2012; Duman and Gul, 2013). Although the variance in absolute and relative fecundity between individuals of the same population in the year, appeardivers reproductive strategy for different species and stocks, which has been attributed to relation of influential factors to modify potential fecundity (Yoneda and Wright, 2004; Thorsen et al., 2006).
Statistical analysis in the present work exhibit highly significant correlation between reproductive and morphometric parameters. Table 2 showed that fecundity is highly related with length, weight and ovary weight at P<0.01 except of egg diameter included a weak correlation (Figure 2), and could be influenced by food abundances, environmental conditions, spawning strategy and reproductive pattern (Lambert, 2008). Our present study revealed match with previous studies in the general directions on present species and other species of cyprinid (Al-Saleh et al., 2010; Oymak et al., 2011; Al-Saleh et al., 2012; Al-Noor and Abdullah, 2015).
The results shown agree in related eggs diameters (1.624-2.015 mm) with Shafi and Jasim (1982) in Habanya and Tharthar lakes, when they recorded 1.6-1.7 mm and with Al-Selah et al. (2010) in the middle sector of Euphrates River in Syria when they found eggs diameters between 1.6-2.0 mm, our observation in eggs diameters differed from the results of Al-Saleh et al. (2012) who's found eggs diameters ranged between 1.56-1.92 mm, but disagree with Duman and Gul (2013) in mean of eggs diameters was 1.021 mm in April, this could be due to localities variation, environmental factors, food availability, fish size (Bagenal, 1978). Eggs size is important parameter to success the reproductive cycle of species that strongly associated with maximum size of the species (genetic), physiology, food available and environmental conditions. Caloric value of oocyte yolk which is largely associated of content water, lipids and protein that enables the larvae to pass the critical stage in their life history (Kamler, 2005). Yolked oocyte diameter is largely governed by the natural size of species attained, environmental conditions, reproductive strategy, spawning pattern and parental care (Lambert et al., 2003).
Elative condition factors is a common parameter used to measured fish status. Covariance in relative condition factor associated with ovary development, feeding intensity, age, physiological of fish and with increasing the weight Mat Isa et al. (2010). The condition factors somewhat vary from place to another and from time overall the year (Froese, 2006).
Our observations in relative condition factor of L. vorax female compatible with Shafi and Jasim (1982) in Habbaniyah and Tharthar lakes in Iraq and study of Oymak et al. (2011) in Ataturk Dam lake in Turkey, while higher than Al-Saleh et al. (2012) was (kn=0.71) in the middle reaches of Euphrates River in Syria and Duman and Gul (2013) (Kn=0.865) in Karakaya reservoir in Euphrates River, Turkey.
Values of relative condition factor started to increase gradually from November 2016 to attain the higher in January 2017 (Figure 3), due to gonads development and declined from February to April, it could be to the spawning, then values raised from May to July, this rising attributed to feeding, moreover, the variations in relative condition factor influenced by gonad development, nutritional conditions, water temperature (Doddamani et al., 2001; Froese, 2006; Oymak et al., 2011; Al-Saleh et al., 2012; Duman and Gul, 2013).
4 Conclusion
The results shown that there is one peak in the reproductive strategy of L. vorax in Huwaiza marsh, which has been lying-in early before other species (Hashim et al., 1984; Kahkesh et al., 2010; Mortezavizadeh et al., 2010; Najim et al., 2012; Al-Noor and Abdullah, 2015; Mohamed et al., 2017), that inhabits in the same habitat to enable the new generation of small fish for represented species to feeding on larvae and other small fish species (Hussain and Ali, 2006; Hussain et al., 2009; Mohamed et al., 2015) The results revealed high fecundity of investigated species, therefore few number of mature female capable of maintenance existences the species.
Authors’ contributions
AJA suggested the manuscript plan and conducted a survey of previous studies, wrote the text of the paper, performed the statistical analysis and participated in collected the samples, calculated of the results, contributions in all laboratory works. FM participated in design the manuscript plan shared in all laboratory biological works and read and approved the final manuscript. FH participated in some works in laboratory and manuscript coordination.
Acknowledgments
The authors are thankful to Marine Science Center and marine vertebrate Department University of Basrah to prepare the laboratories that helped in the preparation of the research.
Alam M., and Pathak J.K., 2010, Assessment of fecundity and gonadosomatic index of commercially important fish Labeo rohita from Ramganga river, Int. J. Pharma., Biosci, 1(3):1-6
Ahmed H.A., Al-Mukhtar M.A., and Al-Adhub H.Y., 1984, The reproductive biology of Carasobarbus luteus (Pisces, Cyprinidae) in Al-Hammar Marsh, Iraq, Cybium, 8: 69-80
Al-Dabical A.Y., and Al-Daham N.K., 1995, The growth of Aspius vorax Heckel in the first year of age at Shatt Al-Basrah Canal, Marina Mesopo., 8: 344-354
Ali M.D., Ali A.M., and Zaki L.M., 1986, The general condition and calorificvalue of the freshwater fish Aspius vorax and Barbus luteus in Al-Tharthar Reservoir, J. of Bio. Sci. Research, 17: 223-230
Al-Noor S.S., and Abdullah A.H.J., 2015, Structural diversity of fish communities in the North part of Shatt Al-Arab River-North of Basrah-Qurna, Basrah J. Agric. Sci., 28(2):15-29 (In Arabic)
Al-Saleh F., Hammoud V., and Al-Hussein A., 2010, Reproduction biology of Aspius vorax(Heckle,1843) within the middle Euphrates River, Damascus University, J. for Basic Sci., 26(1):159-170 (In Arabic)
Al-Saleh F., Hammoud V., Hussein A., and Alhazzaa R., 2012, On the growth and reproductive biology of asp, Aspius vorax, population from the middle reaches of Euphrates River, Turkish J. Fisheries and Aqu. Sci., 12: 149-156
Al-Tameemi R., Al-dubaikal A.,and Salman N.A.,2010, Comparative study of α-amylase activity in three Cyprinid species of different feeding habits from Southern Iraq, Turkish J. of Fisheries and Aqu. Sci., 10: 411-414
Bagenal T.,1978, Methods for the assessment of fish production in freshwaters, 3rd ed. Blackwell Sci. Publ. Oxford, pp.365
Coad B.W., 2010, Freshwater fishes of Iraq, Pensoft Publishers, Sofia, pp.294
Dar S.A., Najar A.M., Balkhi M.H., Rather M.S., and Sharma R., 2012, Length weight relationship and relative condition factor of Schizopyge esocinus(Heckel, 1838) from Jhelum River, Kashmir,International J. Aqu. Sci., 3(1): 29-36
Doddamani M., Rameshaand T.J., and Shanbhogue S.L.,2001, Length-weight relationship and condition factor of Stolephorus bataviensisfrom Mangalore area, Indian J. Fish., 48: 329-332
Dudgeon D., Arthington A.H., Gessner, M.O, Kawabata Z.I., Knowler D.J., Leveque C., Naiman R.J., Prieur-Richard A.H., Soto D., Stiassny M.L., and Sullivan C.A., 2006, Freshwater biodiversity: Importance, threats, status and conservation challenges, Biol. Rev., 81(2): 163‒182
https://doi.org/10.1017/S1464793105006950
Duman E., and Gul M.R., 2013, Age, growth, fecundity and mortality of Aspius vorax(Heckel, 1843) in Karakaya Reservoir (in Euphrates River), Turkey, Ege J. Fish Aqu. Sci., 30(4): 155-159
Epler P., Sololowska-Mikolajczyk M., Popek W., Bieniarz W., Bartel K., and Szczerbowski J.A., 2001, Reproductive biology of selected fish species from Lakes Tharthar and Habbaniya in Iraq, Archive of Polish Fisheries, 9: 199-209
Froese R., 2006, Cube law, condition factor and weight–length relationships: history, meta-analysis and recommendations, J. Appl. Ich., 22: 241-253
https://doi.org/10.1111/j.1439-0426.2006.00805.x
George W.B., 1981, Patterns of parental investment, dispersal and size among coral-reef fishe, Env. Biol. Fish., 6(1): 65-85
https://doi.org/10.1007/BF00001801
Hunter J.R., Macewicz B.J., Chyan-huei Lo N., and Kimbrell C.A., 1992, Fecundity, spawning and maturity of female Dover Sole, Microstomus pacificus, with an evaluation of assumptions and precision, Fish. Bull., 90(1): 101-128
Hussain N.A., and Ali T.S., 2006, Trophic nature and feeding relationships among Al-Hammer marsh fishes, Southern Iraq, Mar. Bull., 1(1): 9-18
Hussain N.A., Saoud H.A., and Al-Shami E.J., 2009,Specialization,competition and diet overlap of fish assemblage in the recently restored Southern Iraqi marshes, Mar. Bull.,4(1): 21-35
Kahkesh F.B., Feshalami M.Y., Amiri F., and Nickpey M., 2010, Effect of Ovaprim, Ovatide, HCG, LHRH-A2, LHRHA2+CPE and carp pituitary in Benni Barbus sharpeyi artificial breeding, Global Veterinaria, 5(4): 209-214
Kamler E., 2005, Parent-egg-progeny relationships in teleost fishes: An energetics perspective, Rev. Fish Bio. Fish., 15(4): 399-421
https://doi.org/10.1007/s11160-006-0002-y
Lagler K.F., 1966, Freshwater Fishery Biology, W.M.C. Brown Company, Iowa, pp.421
Lambert Y., Yaragina N.A., Kraus G., Marteinsdottir G., and Wright P.J., 2003, Using environmental and biological indices as proxies of egg and larval production of marine fish. J. Northw. Atl. Fish. Sci., 33: 115-159
https://doi.org/10.2960/J.v33.a7
Lambert Y., 2008, Why should we closely monitor fecundity in marine Fish population, J. Northw. Atl. Fish. Sci., 41: 93-106
https://doi.org/10.2960/J.v41.m628
Mat Isa M., Rawi C.S, Rosla R., Mohd Shah S.A., and Shah A.S.R., 2010, Length-weight relationships of freshwater fish species in Kerian River basin and Pedu Lake, Research J. of Fisheries and Hydrobio., 5(1): 1-8
Mohamed A.R.M., Hussein S.A., and Mutlak F.M., 2015, The feeding relashinships of six fish species in East Hammar marsh Southern Iraq.Thi-Qar Univ. J. for Agric. Res. 4(1): 460-477 (In Arabic)
Mohamed A.R.M., Hussein S.A., and Mutlak F.M., 2017, Some biological aspects of four fish species in East Hammar Marsh, Iraq. J. of Scientific and Engineering Research, 4(8): 278-287
https://doi.org/10.14299/ijser.2017.03.003
Mortezavizadeh S.A., Feshalami Y.M., and Kahkesh F.B., 2010, Effect of GnRHa (Ala6,des-Gly10 mGnRHa), LHRH-a (desGly10, [D-ala6]LH-LHEthylamide and carp pituitary inartificial propagation of Gattan, Barbus xanthopterus (Heckel,1843), World J. Fish and Marine Sci., 2(4): 280-284
Murua H., Kraus G., Saborido-Rey F., Witthames P.R., and Junquera S., 2003, Procedures to estimate fecundity of marine fish species in relation to their reproductive strategy, J. Northw. Atl. Fish. Sci., 33: 33-54
Najim S.M., Al-Mudhaffar R.A.A., and Jassim F.K., 2012, Some reproductive characters of fantail goldfish Carassius auratus auratusfemales from rearing ponds in Basrah, Southern Iraq, Iraqi J. Aquacult., 9(1): 83-94
Nikolsky G.V., 1969, Theory of fish population dynamics, Otto Science Publishers, Koenigstein, pp.317
Oymak S.A., Ünlü E., Parmaksiz A., and Dogan N., 2011, A study on the age, growth and reproduction of Aspius vorax (Heckel, 1843) (Cyprinidae) in Atatürk Dam Lake (Euphrates River), Turkey, Turk. J. Fish. Aqu. Sci., 11: 217-225
Pankhurst N.W., and King H.R., 2010, Temperature and salmonid reproduction: implications for aquaculture, J. Fish Biol., 76: 69-85
https://doi.org/10.1111/j.1095-8649.2009.02484.x
Shafi M., and Jasim B.M., 1982, Some aspects of the biology of a cyprinid, Aspius vorax (Heckel 1834), J. of Fish Bio., 20: 271-278
https://doi.org/10.1111/j.1095-8649.1982.tb04708.x
Szypuła J., Epler P., Bartel R., and Szczerbowski J.A., 2001, Age and growth of fish in lakes Tharthar, Razzazah, and Habbaniya, Arch. Polish Fish, 9: 185-197
Thorsen A., Marshall C.T., and Kjesbu O.S., 2006, Comparison of various potential fecundity models for north-east Arctic cod Gadus morhua, L. using oocyte diameter as a standardizing factor, J. Fish Biol., 69: 1709-1730
https://doi.org/10.1111/j.1095-8649.2006.01239.x
Wright P.J., and Trippel E.A., 2009, Fishery-induced demographic changes in the timing of spawning: consequences for reproductive success, Fish and Fisheries, 10: 283-304
https://doi.org/10.1111/j.1467-2979.2008.00322.x
Yoneda M., and Wright P.J., 2004, Temporal and spatial variation in reproductive investment of Atlantic cod Gadus morhuain the Northern North Sea and Scottish west coast, Mar. Ecol. Prog. Ser., 276: 237-248
. PDF(470KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Abdul Hussein Jaafer Abdullah
. Faleh Musa Al-Zaidy
. Fawziah Sh. Habbeb
Related articles
. Leuciscus vorax
. Reproductive characteristics
. Huwaiza marsh
. Southern Iraq
Tools
. Email to a friend
. Post a comment
.png)
.png)
.png)
.png)


